J Vet Sci.
2007 Jun;8(2):139-145. 10.4142/jvs.2007.8.2.139.
Diagnosis of Helicobacter spp. infection in canine stomach
- Affiliations
-
- 1Department of Pathology, Faculty of Veterinary Science, Chulalongkorn University, Bangkok 10330, Thailand. achariya.sa@chula.ac.th
- 2Department of Veterinary Public Health, Faculty of Veterinary Science, Chulalongkorn University, Bangkok 10330, Thailand.
- 3Department of Microbiology, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand.
- KMID: 1089664
- DOI: http://doi.org/10.4142/jvs.2007.8.2.139
Abstract
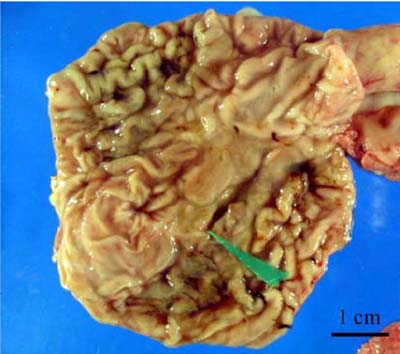
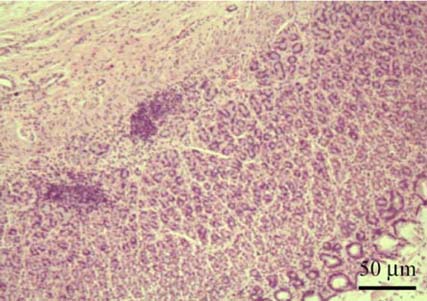
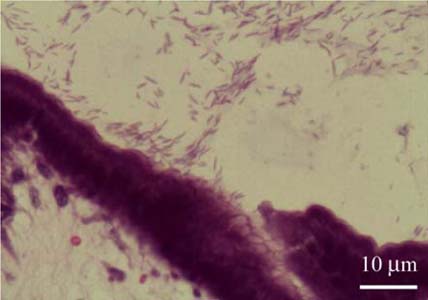
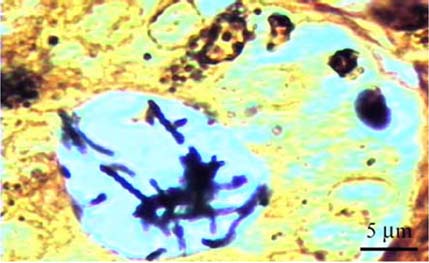
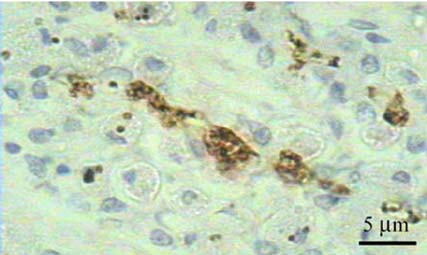
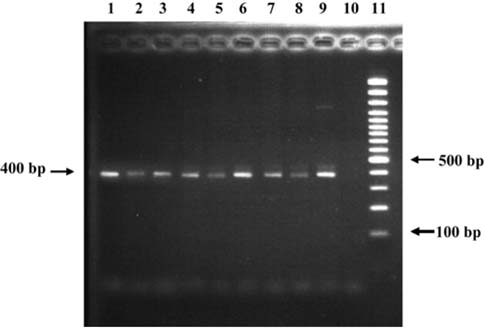
- A total of 75 biopsied samples of cardia, fundus, body, and pyloric antrum from necropsied dogs that were submitted to the Department of Pathology, Faculty of Veterinary Science, Chulalongkorn University from April 2003 to June 2004 were investigated. The objectives of this study were to determine the prevalence of Helicobacter spp. in canine stomach by polymerase chain reaction (PCR) in comparison to histochemistry versus immunohistochemistry (IHC), and to correlate these diagnostic methods with the clinical significance in infected dogs. Histopathological results revealed 60.0% (45/75) of samples to be positive, and consisted of mild gastritis in 64.44% (29/45), moderate gastritis in 11.11% (5/45), and severe gastritis in 24.44% (11/45). The proportion showing no histopathological lesions was 40.0% (30/75). Helicobacter spp. were localized to the luminal crypt in 18.67% (14/75), gastric pit in 22.67% (17/75), gastric gland in 21.33% (16/75), and gastric epithelium in 8% (6/75). The percentages of positive samples of Helicobacter spp. diagnosed by hematoxylin and eosin stain (H&E), Warthin Starry stain (WSS), IHC with rabbit polyclonal anti-H. pylori antibody, and PCR were 17.3% (13/75), 46.7% (35/75), 30.7% (23/75), and 10.7% (8/75), respectively. No significant differences weree observed in histopathological changes in portions of the stomach (p > 0.05). The diagnosis of Helicobacter spp. by PCR in comparison to that by WSS and IHC was not significantly different (p > 0.05). There were no relationships between pathological studies using H&E, WSS, and IHC, and especially between PCR and clinical signs of Helicobacter spp. infections in canine stomachs (p > 0.05). The present study revealed significantly different levels of correlation for Helicobacter spp. detection between H&E and WSS (p < 0.001). Results indicate that the method of choice for diagnosis of Helicobacter spp. infection in canine stomach is dependent on the purpose of study and appropriate specimen collection.
Keyword
MeSH Terms
-
Animals
DNA, Bacterial/chemistry/genetics
Dog Diseases/*diagnosis/epidemiology/*microbiology
Dogs
Female
Gastric Mucosa/microbiology
Gastritis/diagnosis/epidemiology/microbiology/*veterinary
Helicobacter/genetics/*isolation & purification
Helicobacter Infections/diagnosis/epidemiology/microbiology/*veterinary
Immunohistochemistry/veterinary
Male
Polymerase Chain Reaction/veterinary
Figure
Reference
-
1. Ashton-Key M, Diss TC, Isaacson PG. Detection of Helicobacter pylori in gastric biopsy and resection specimens. J Clin Pathol. 1996. 49:107–111.
Article2. Buczolits S, Hirt R, Rosengarten R, Busse HJ. PCR-based genetic evidence for occurrence of Helicobacter pylori and novel Helicobacter species in the canine gastric mucosa. Vet Microbiol. 2003. 95:259–270.
Article3. Buczolits S, Rosengarten R, Hirt R, Busse HJ. Classification of a Brevundimonas strain detectable after PCR with a Helicobacter-specific primer pair. Syst Appl Microbiol. 2001. 24:368–376.
Article4. Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clin Microbiol Rev. 1997. 10:720–741.
Article5. Eaton KA, Radin MJ, Kramer L, Wack R, Sherding R, Krakowka S, Fox JG, Morgan DR. Epizootic gastritis associated with gastric spiral bacilli in cheetahs (Acinonyx jubatus). Vet Pathol. 1993. 30:55–63.
Article6. Eaton KA, Dewhirst FE, Paster BJ, Tzellas N, Coleman BE, Paola J, Sherding R. Prevalence and varieties of Helicobacter species in dogs from random sources and pet dogs: animal and public health implications. J Clin Microbiol. 1996. 34:3165–3170.
Article7. Esteves MI, Schrenzel MD, Marini RP, Taylor NS, Xu S, Hagen S, Feng Y, Shen Z, Fox JG. Helicobacter pylori gastritis in cats with long-term natural infection as a model of human disease. Am J Pathol. 2000. 156:709–721.
Article8. Flatland B. Helicobacter infection in humans and animals. Comp Cont Educ Pract Vet. 2002. 24:688–696.9. Fox JG. The non-H. pylori helicobacters: their expanding role in gastrointestinal and systemic diseases. Gut. 2002. 50:273–283.
Article10. Fox JG, Correa P, Taylor NS, Lee A, Otto G, Murphy JC, Rose R. Helicobacter mustelae-associated gastritis in ferrets. An animal model of Helicobacter pylori gastritis in humans. Gastroenterology. 1990. 99:352–361.
Article11. Fox JG, Lee A. The role of Helicobacter species in newly recognized gastrointestinal tract diseases of animals. Lab Anim Sci. 1997. 47:222–255.12. Geyer C, Colbatzky F, Lechner J, Hermanns W. Occurrence of spiral-shaped bacteria in gastric biopsies of dogs and cats. Vet Rec. 1993. 133:18–19.
Article13. Hall JA. Ettinger SJ, Feldman EC, editors. Diseases of the stomach. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat. 2000. 5th ed. Philadelphia: Saunders;1154–1181.14. Handt LK, Fox JG, Stalis IH, Rufo R, Lee G, Linn J, Li X, Kleanthous H. Characterization of feline Helicobacter pylori strains and associated gastritis in a colony of domestic cats. J Clin Microbiol. 1995. 33:2280–2289.
Article15. Happonen I, Saari S, Castren L, Tyni O, Hanninen ML, Westermarck E. Occurrence and topographical mapping of gastric Helicobacter-like organisms and their association with histological changes in apparently healthy dogs and cats. Zentralbl Veterinarmed A. 1996. 43:305–315.
Article16. Henry GA, Long PH, Burns JL, Charbonneau DL. Gastric spirillosis in beagles. Am J Vet Res. 1987. 48:831–836.17. Herbrink P, van Doorn LJ. Serological methods for diagnosis of Helicobacter pylori infection and monitoring of eradication therapy. Eur J Clin Microbiol Infect Dis. 2000. 19:164–173.
Article18. Hermanns W, Kregel K, Breuer W, Lechner J. Helicobacter-like organisms: histopathological examination of gastric biopsies from dogs and cats. J Comp Pathol. 1995. 112:307–318.
Article19. Lage AP, Godfroid E, Fauconnier A, Burette A, Butzler JP, Bollen A, Glupczynski Y. Diagnosis of Helicobacter pylori infection by PCR: comparison with other invasive techniques and detection of cagA gene in gastric biopsy specimens. J Clin Microbiol. 1995. 33:2752–2756.
Article20. Leodolter A, Wolle K, Malfertheiner P. Current standards in the diagnosis of Helicobacter pylori infection. Dig Dis. 2001. 19:116–122.
Article21. Luna LG. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 1968. 3rd ed. New York: McGraw-Hill;238–240.22. Monteiro L, de Mascarel A, Sarrasqueta AM, Bergey B, Barberis C, Talby P, Roux D, Shouler L, Goldfain D, Lamouliatte H, Mégraud F. Diagnosis of Helicobacter pylori infection: noninvasive methods compared to invasive methods and evaluation of two new tests. Am J Gastroenterol. 2001. 96:353–358.
Article23. Pirarat N, Makbunsri T, Sukkamon S, Amornchailertrat S, Rungsipipat A, Sunyasootcharee B. The relationship between pathological gastric changes and Helicobacter spp. in dog. Thai J Vet Med. 2003. 33:73–80.24. Ploskonosova II, Baranov VI, Gaziev AI. Estimation of DNA damage and repair in tissues of gamma-irradiated animals using the polymerase chain reaction. Biochemistry (Mosc). 1999. 64:1320–1325.25. Queiroz DM, Rocha GA, Mendes EN, De Moura SB, De Oliveira AM, Miranda D. Association between Helicobacter and gastric ulcer disease of the pars esophagea in swine. Gastroenterology. 1996. 111:19–27.
Article26. Riley LK, Franklin CL, Hook RR Jr, Besch-Williford C. Identification of murine helicobacters by PCR and restriction enzyme analyses. J Clin Microbiol. 1996. 34:942–946.
Article27. Simpson KW, Strauss-Ayali D, McDonough PL, Chang YF, Valentine BA. Gastric function in dogs with naturally acquired gastric Helicobacter spp. infection. J Vet Intern Med. 1999. 13:507–515.
Article28. Vaira D, Holton J, Menegatti M, Ricci C, Gatta L, Geminiani A, Miglioli M. Review article: invasive and non-invasive tests for Helicobacter pylori infection. Aliment Pharmacol Ther. 2000. 14:Suppl 3. 13–22.29. Yamasaki K, Suematsu H, Takahashi T. Comparison of gastric lesions in dogs and cats with and without gastric spiral organisms. J Am Vet Med Assoc. 1998. 212:529–533.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Helicobacter pylori Eradication in Patients Undergoing Gastrectomy: Diagnosis and Therapy
- Detection of Helicobacter spp. in gastric, fecal and saliva samples from swine affected by gastric ulceration
- Microbial Profile of the Stomach: Comparison between Normal Mucosa and Cancer Tissue in the Same Patient
- Diagnosis and Treatment of Helicobacter Pylori Infection
- The Association between Helicobacter pylori Infection and Gastric Adenocarcinoma: a Review of the Literature